Acids and Bases
classes of chemical compounds.
Generally substances are called acids if they contain hydrogen (HCl, HNO3, H2SO4, CH3COOH) and dissociate in water to form H+ ions (more accurately, hydronium ions, H3O+). The presence of these ions determines the sharp taste characteristic of aqueous acid solutions and their ability to change the color of chemical indicators. Acids are classified, by the number of replaceable protons, as monobasic (for example, nitric acid, HNO3; hydrochloric acid, HCl; acetic acid, CH3COOH), dibasic (sulfuric acid, H2SO4; carbonic acid, H2CO3), and tribasic (orthophosphoric acid, H3PO4). The more hydronium ions present in the aqueous acid solution, that is, the higher the degree of dissociation, the stronger the acid. Acids that undergo complete dissociation in dilute solutions are called strong acids. Weak acids have an ionization constant (characterizing the degree of dissociation of the acid in solution, for example, at 25°C) of less than 10–5 (acetic acid, 1.8 × 10–5; prussic acid 7.9 × 10–10). The dissociation of polybasic acids occurs in several stages, each of which is characterized by a particular ionization constant. For example, the ionization constant for the dissociation of H3PO4 into H+ and H2PO4– is 7 × 10–3; for H2PO4– into H+ and HPO42–, 8 × 10–8; and for HPO42– into H+ and PO43–, 4.8 × 10–13.
Generally substances are called bases if they contain the hydroxyl group OH [KOH, NaOH, Ca(OH)2] and undergo dissociation in aqueous solution to form hydroxyl OH– ions. The majority of bases are insoluble in water. Water-soluble bases are known as alkalis. The presence of OH– ions explains the caustic taste characteristic of alkaline solutions and their ability to change the color of chemical indicators. Bases containing one, two, or three hydroxyl groups are referred to as monoacidic, diacidic, and triacidic, respectively. Bases that do not undergo complete dissociation when dissolved in water are called weak bases. Examples of strong bases are potassium hydroxide, KOH; sodium hydroxide, NaOH; and barium hydroxide, Ba(OH)2.
The concept of acids and bases emerged at the very beginning of the study of chemistry. In 1778 the French chemist A. L. Lavoisier attempted to explain the characteristic features of acids by relative oxygen content. This concept proved unjustified when it became apparent that many oxygen-containing substances (oxides of metals, alkalies, salts) do not exhibit acidic properties and that a number of typical acids (hydrochloric, prussic, hydrofluoric) do not contain oxygen at all. This was demonstrated by the English scientist H. Davy in 1810 and by the French scientist J. L. Gay-Lussac in 1814. The Swedish chemist J. J. Berzelius proposed (1812–19) that acidic and basic properties were due to the electrical character of the oxides—that is, he regarded the electronegative oxides of nonmetals (and certain metals, such as chromium and manganese) as acids and electropositive metal oxides as bases. In 1814, Davy suggested hydrogen as the carrier of acidic properties, since it was a constituent of all compounds known at the time to exhibit those properties. The German chemist J. von Liebig refined this concept substantially in 1833 by stating that the acidic properties of a substance are not determined by the total number of hydrogen atoms contained but only by those atoms that can be replaced by a metal to form salts. After the appearance of the electrolytic dissociation theory of the Swedish scientist S. Arrhenius (1884–87), those compounds were called acids that formed hydrogen H+ ions upon dissociation in aqueous solution, and those that dissociated with the detachment of the hydroxyl OH– ion were called bases. The development of the solution theory demonstrated that the interaction both of the substances themselves and of their dissociation products with a solvent plays an important role in electrolytic dissociation. It was also found that the H+ ion cannot exist in a free state in solution. Because of its very high charge density the H+ ion combines stably with the molecules of the solvent (solvation) and actually exists in the form of a solvated ion; in aqueous solutions the H+ actually exists as hydronium ion, which is also a carrier of acidic properties.
A definition of the concepts of acid and base on the basis of the electrolytic dissociation theory is often fully sufficient for practical purposes. However, as has long been established, many compounds exhibiting typically acidic and basic properties contain neither hydrogen nor the hydroxyl group. Furthermore, the same substance frequently behaves as an acid in certain reactions and as a base in others. The ability of a substance to react as either acid or base does not therefore appear to be an absolute property of the substance but is expressed in specific chemical reactions related to the acid-base class. In these reactions, one of the interacting substances acts as an acid in relation to the other substance, which acts as a base. Therefore, the ability of a substance to behave as either acid or base is a functional characteristic. Numerous attempts have been made to develop a unified theory that would make it possible, taking into account the conditions mentioned, unequivocally to call a given substance acid or base. However, no single criterion has yet been found.
The two most popular concepts are those developed by the Danish physical chemist J. N. Brønsted and by the American physical chemist G. N. Lewis (1923). Brønsted classifies as acids hydrogen-containing substances that give up positive hydrogen ions, that is, protons (proton, or Brønsted, acids), and as bases, substances that accept protons. According to Brønsted, both neutral molecules and ions can fulfill the functions of both acids and bases. A chemical reaction involving proton transfer from acid to base (AH + B– ⇄ A– + BH, where AH is an acid and B– is a base) is called an acid-base, or protolytic, reaction. Since protolytic reactions are reversible (proton transfer takes place in both the forward reaction and in the reverse reaction), the products of the forward reaction also fulfill the functions of acid and base (conjugate acids and bases) with respect to each other—that is, BH = acid, and A– = base. For example, in the reaction H2SO4 + H2O ⇄ HSO4– + H3O+, the acids are H2SO4 and H3O+, and the bases, HSO4– and H2O. The Brønsted concept offers a precise criterion for relating chemical reactions to the acid-base type. It makes it possible to express the basic characteristics of protolytic equilibriums in quantitative form and to arrange hydrogen-containing substances in a series of increasing ability to give up a proton—according to acidity.
These merits of the protolytic equilibrium theory made for its predicted strength and ensured the broad application of Brønsted’s concepts in practical chemistry. At the same time, these concepts are inherently limited, in that linking the acidic properties of a substance to the presence in it of hydrogen still excludes the large number of acidic substances that contain no hydrogen. Electron-unsaturated compounds (for example, boron, aluminum, and tin halides) and certain metal oxides are among this group, known as aprotonic, or Lewis, acids. According to Lewis’ concept, which to an extent makes up for the above deficiency, an acid is a substance that accepts an electron pair in a chemical reaction and a base is a substance that gives up an electron pair. As a result, the electron unsaturation of an acid molecule is filled by electrons from the base. In addition, a new compound (salt) is formed with a stable electron shell (in particular, an octet) and a donor-acceptor bond. For example,
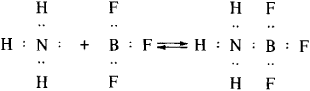
where BF3 is the acid and NH3 the base.
According to Lewis, an important feature of acid-base reactions is the collectivization of the electron pair of the base. This distinguishes acid-base reactions from oxidation-reduction reactions, which involve the complete removal (one or more at a time) of electrons from the molecules of the reducing agent by the molecules of the oxidizer-carrier; no collectivized orbits result from the process. Unlike Brønsted, Lewis associates acid-base properties not with the presence of specific chemical elements (hydrogen, in particular) but with the structure of the outer electron shells of the atoms alone. At the same time, there is an interrelation between the two concepts, in that a strong affinity for an electron pair is characteristic of both the H+ ion and the Lewis acid. There are certain other concepts about acids and bases but they are not widely accepted.
Both the Brønsted theory and the Lewis theory have found wide practical application. A change in acidity or basicity of a medium is often used to increase the reaction rate and to alter the reaction mechanism; this forms the basis of acid-base catalysis and is used widely in the chemical industry. It should be especially noted that Brønsted and Lewis acids in many cases exhibit similar catalytic activity. Acid-base processes have found wide application in the chemical industry (neutralization, hydrolysis, metal etching). Many acids (sulfuric, nitric, hydrochloric, orthophosphoric) and alkalies (caustic potash, caustic soda) are important both as starting materials and as products in the major branches of the chemical industry.
Acids and bases fulfill diverse structural and dynamic functions in living organisms by taking part in many biological processes. As a rule, these processes are highly sensitive to the acidity or basicity of the medium. The directional effect produced by acids and bases has found application in medicine. For example, weak solutions of hydrochloric acid are used to in-crease gastric secretion, and weak boric acid solution is used as a disinfectant and astringent in mouthwashes. On the other hand, the penetration of concentrated acids and bases into the organism may cause serious burns to internal organs, reduction in cardiac activity, and other damage, frequently resulting in death.
REFERENCE
Luder, W., and S. Zuffante. Elektronnaia teoriia kislot i osnovanii. Moscow, 1950. (Translated from English.)Usanovich, M. I. Chto takoe kisloty i osnovaniia. Alma-Ata, 1953.
Pauling, L. Obshchaia khimiia. Moscow, 1974. (Translated from English.)
Kratkaia khimicheskaia entsiklopediia, vol. 2. Moscow, 1963.
IA. M. VARSHAVSKII