glycogen
Glycogen
The primary reserve polysaccharide of the animal kingdom. It is found in the muscles and livers of all higher animals, as well as in the cells of lower animals. Because of its close relationship to starch, it is often called animal starch, although glycogen is found in some lower plants, fungi, yeast, and bacteria. See Starch
Glycogen is a nonreducing, white, amorphous polysaccharide which dissolves readily in cold water, forming an opalescent, colloidal solution. The molecular weight of glycogen is usually very high, and it varies with the source and the method of preparation; molecular weights of the order of 1-20 × 106 have been reported. Chemical studies show glycogen to possess a branched structure similar to the amylopectin starch fraction.
In its biochemical reactions, glycogen is similar to starch. It is attacked by the same plant amylases that attack starch, and like starch, it is degraded to maltose and dextrins. Both glycogen and starch are broken down by animal or plant phosphorylase enzyme in the presence of inorganic phosphate with the production of α- d -glucose-1-phosphate. See Carbohydrate metabolism
The metabolic formation of glycogen from glucose in the liver is frequently termed glycogenesis. In fasted animals, glycogen formation can be induced by the feeding, not only of materials that can be hydrolyzed to glucose and other monosaccharides, such as fructose, but also of various other materials. A number of l -amino acids, such as alanine, serine, and glutamic acid, upon deamination in the liver give rise to substances, such as pyruvic acid and α-ketoglutaric acid, that can be converted in the liver to glucose units which are subsequently converted to glycogen. Furthermore, substances such as glycerol derived from fats, dihydroxyacetone, or lactic acid can all be utilized for glycogen synthesis in the liver. Such noncarbohydrate precursors are termed glycogenic compounds. The process of glycogen formation from these precursors is known as glyconeogenesis. The term glycogenolysis is used to connote glycogen breakdown. See Polysaccharide
glycogen
[′glī·kə·jən]Glycogen
(also called animal starch), (C6H10O5)n, the basic reserve carbohydrate of animals and man; also found in some bacteria, yeasts, and fungi. Its content is particularly high in the liver (3-5 percent) and muscles (0.4-2 percent). Glycogen was discovered by the French physiologist

C. Bernard in the liver (1857). Glycogen is a homopolysaccharide consisting of 6,000-20,000 or more α-D-glucose radicals. The glycogen molecule has a branched structure; the average length of the unbranched chain is 10-14 glucose radicals (see Figures 1 and 2). The molecular weight of glycogen is 105-107.
Glycogen is a white amorphous powder that is polydisperse
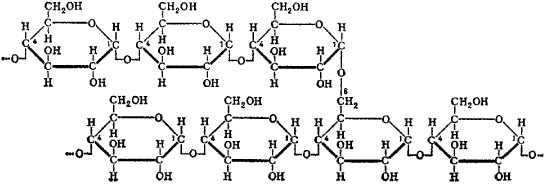
and opalescent in solution. It is optically active ([α]D = + 198°). Glycogen solutions containing iodine exhibit colors varying from violet-brown to violet-red. Glycogen is split in two ways in the organism. Hydrolytic splitting of the glycogen contained in food occurs during the digestive process with the participation of amylases. The processes starts in the oral cavity and ends in the small intestine (at pH 7-8), leading to the formation of dextrins and then of maltose and glucose. Glucose enters the bloodstream, and the excess glucose participates in the synthesis of glycogen, in the form of which it is deposited in the tissues. Hydrolytic splitting of glycogen is also possible in tissue cells, but this process is of lesser importance. The main path of intracellular glycogen transformation is phosphorolytic splitting, which occurs under the influence of phosphorylase and leads to sequential splitting off of glucose units from the glycogen molecule, accompanied by their simultaneous phosphorylation. The glucose-1-phosphate formed in this case, may be included in the glycogenolysis process. The phosphorylation of glucose is a mandatory stage of glycogen synthesis. The synthesis takes place with the participation of the enzyme glycogen synthetase. Cytoplasm contains glycogen in the form of polysaccharides of various molecular weights and with a variety of physicochemical properties. The composition of glycogen may vary depending on the functional state of the tissue, the season of the year, and other factors.
The glycogen content of tissues depends on the activity ratio of phosphorylase and glycogen synthetase, as well as on the tissue’s glucose supply from the blood. Lowering the blood sugar leads to a high phosphorylase activity, and so-called glycogen mobilization takes place, accompanied by its disappearance from the cytoplasm. Conversely, glycogen synthesis predominates in cases of enrichment of the blood with glucose (for example, after the intake of food). An important function in maintaining a constant blood-sugar level is performed by the liver, which either transforms the glucose excess into glycogen or mobilizes the excess glucose in case of a sugar deficiency in the blood. Other organs store glycogen for their own consumption. In this case, glucose entering the cell is usually utilized in the synthesis of glycogen, which is subsequently consumed as the basic substrate in anaerobic carbohydrate transformations. An important role in regulating the blood-sugar content is played by the central nervous system. The brain tissue contains little glycogen, and therefore fluctuations in the blood-sugar level are reflected in the metabolic processes in the brain. The direction of glycogen exchange in the liver is controlled by biologically active materials with the participation of the hypothalamus and the sympathetic nervous system. The most important are the hormones adrenalin and glucagon (which cause the mobilization of glycogen), as well as insulin, which stimulates its synthesis.
REFERENCE
Khimiia uglevodov. Moscow, 1967.A. A. BOLDYREV