feldspar
, felsparfeldspar
[′fel‚spär]feldspar
Feldspar
the most widely distributed group of rock-forming minerals, constituting more than 50 percent of earth and lunar rocks; they are also found in meteorites. The composition of feldspars is basically determined by the component ratio in a ternary system: NaAlSi3O8—KAlSi3O8—CaAl2Si2O8; that is, feldspars are aluminosilicates of Na, K, and Ca, with admixtures of Ba, Sr, Pb, Fe, Li, Rb, Cs, Eu, Ce, and other elements. All feldspars have a basic three-dimensional framework composed of tetrahedral (Al, Si)O4 groups in which one-third to one-half of the Si atoms are replaced by Al. Univalent K+ and Na+ cations, with an Al/Si ratio of 1:3, or bivalent Ca2+ and Ba2+ cations, with an Al/Si ratio of 1: 2, are arranged in the large vacancies within this framework.
Two series of solid solutions are differentiated in the feldspar group: anorthoclases, or alkali feldspars (KAISi3O8—NaAlSi3O8), and plagioclases (NaAlSi3O8–CaAl2Si2O8). The barium feldspar BaAl2Si2O8, known as celsian, is rare, as are solid solutions with compositions between KAlSi3O0 and BaAl2Si2O8, known as hyalophane and containing up to 10–30 percent Ba.
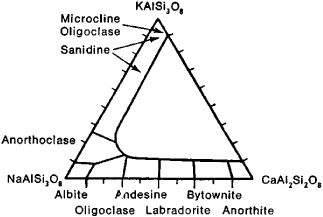
Many feldspar varieties result from complex variation in composition (see Figure 1), the ordering of Al and Si distribution according to structural position, the decomposition of solid solutions, and submicroscopic twinning. The following are examples of potassium feldspars: (1) sanidine, with monoclinic symmetry and disordered Si and Al distribution; (2) maximum microcline (triclinic), with fully ordered Si and Al distribution; (3) intermediate microclines; and (4) orthoclase (assumed to be pseudomonoclinic), composed of submicroscopic twinned triclinic domains.
High-temperature anorthoclases are disordered and form a continuous series of solid solutions. Low-temperature anorthoclases decompose to yield perthites—regular intergrowths of microcline or orthoclase—and sodium feldspars, or albite. All plagioclase varieties are high-temperature (disordered with respect to Al and Si distribution), low-temperature (ordered), or intermediate.
Changes in the degree of ordering and the composition of plagioclases occur with the retention of triclinic symmetry with extremely complex structural changes and the formation of two unmixed regions, which in many oligoclases and labradorites is accompanied by iridescence.
Precise determination of the composition and structural state (ordering) of feldspars is carried out by means of optical orientation diagrams and diagrams of optic axial angles, measured on a Fedorov, or universal, stage, as well as by X-ray methods (diffractometry).
Plagioclases and microclines are nearly always polysynthetically twinned; that is, they form microscopic intergrowths of several elements in accordance with various characteristic laws of twinning.
The tabular or prismatic habit of feldspars in rocks is determined by well-developed {010} and {001} faces, along which perfect cleavage is formed at a right or nearly right angle, as well as by {110} faces. Feldspars have a hardness of 6–6.5 on Mohs’ scale and a density of 2,500-2,800 kg/m3. They have no color of their own; the varied coloration (gray, pink, red, green, black) is due to the presence of very fine inclusions of hematite, iron hydroxides, hornblende, pyroxene, and other minerals; the bluish green color of amazonite and the green color of microcline are associated with the electron center of Pb, substituting for K. Bands of Pb2+, Fe3+, Ce3+, and Eu2+ are distinguished in the luminescence spectra of feldspars. Electron paramagnetic resonance spectra of feldspars are used to determine the electron centers Ti3+ and the hole centers Al—O-—A1, formed through the entrapment of an electron or hole, respectively, by lattice defects.
Feldspars serve as the basis for rock classification. The most important types of rocks are chiefly composed of feldspars: (1) intrusive rocks—granites, syenites (alkali feldspars and plagioclases), gabbros, and diorites (plagioclases); (2) effusive rocks—andesites and basalts; and (3) metamorphic rocks—gneiss, crystalline schists, contact- and regional-metamorphosed rocks, and pegmatites. In sedimentary rocks, feldspars occur as fragmented grains and new formations (authigenic feldspars). Only plagioclases are present in lunar rocks (lunar basalts, gabbros, anorthosites).
Because of the wide variations in composition and properties, feldspars are valuable in the geological and petrographie studies of magmatic and metamorphic rock masses. The isotope 40.K/40Ar ratio of anorthoclases is used to determine the absolute age of rocks.
Alkali feldspars in pegmatites and small-veined rocks are used in the production of ceramics, glass, porcelain, and faience. Feldspar rocks (labradorites) are used as facing material. Amazonite and moonstone (iridescent oligoclase) are decorative stones.
REFERENCES
Deer, W. A., R. A. Howie, and J. Zussmann. Porodoobrazu-iushchie mineraly, vol. 4. Moscow, 1966. (Translated from English.)Marfunin, A. S. Polevye shpaty—fazovye vzaimootnosheniia, opticheskie svoistva, geologicheskoe raspredelenie. Moscow, 1962.
A. S. MARFUNIN