tetryl
[′te·trəl] (organic chemistry)
(NO2)3C6H2N(NO2)CH3 A yellow, water-insoluble, crystalline explosive material melting at 130°C; used in explosives and ammunition. Also known as tetralite.
McGraw-Hill Dictionary of Scientific & Technical Terms, 6E, Copyright © 2003 by The McGraw-Hill Companies, Inc.
The following article is from The Great Soviet Encyclopedia (1979). It might be outdated or ideologically biased.
Tetryl
(or 2, 4, 6-trinitrophenylmethylnitramine), a white crystalline substance that yellows upon exposure to light. Tetryl melts at 129.5°C and has a density of 1.73 g/cm3. It is insoluble in water but dissolves readily in benzene, acetone, and dichloroethane.
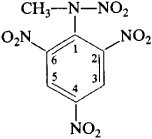
Tetryl is obtained by nitration of the sulfate salts of N-methylaniline, N, N-dimethylaniline, or 2,4-dinitro-N-methylaniline. It is a high explosive, with a detonation rate of 7,500 m/sec at a density of 1.63 g/cm3 and a heat of explosion of 4,609 kilojoules per kg, or 1,100 kilocalories per kg. It is used in primers and as a booster explosive.
The Great Soviet Encyclopedia, 3rd Edition (1970-1979). © 2010 The Gale Group, Inc. All rights reserved.