nitrogen cycle
Nitrogen cycle
The collective term given to the natural biological and chemical processes through which inorganic and organic nitrogen are interconverted. It includes the process of ammonification, ammonia assimilation, nitrification, nitrate assimilation, nitrogen fixation, and denitrification.
Nitrogen exists in nature in several inorganic compounds, namely N2, N2O, NH3, NO2-, and NO3-, and in several organic compounds such as amino acids, nucleotides, amino sugars, and vitamins. In the biosphere, biological and chemical reactions continually occur in which these nitrogenous compounds are converted from one form to another. These interconversions are of great importance in maintaining soil fertility and in preventing pollution of soil and water.
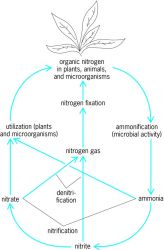
An outline showing the general interconversions of nitrogenous compounds in the soil-water pool is presented in the illustration. There are three primary reasons why organisms metabolize nitrogen compounds: (1) to use them as a nitrogen source, which means first converting them to NH3, (2) to use certain nitrogen compounds as an energy source such as in the oxidation of NH3 to NO2- and of NO2- to NO3-, and (3) to use certain nitrogen compounds (NO3-) as terminal electron acceptors under conditions where oxygen is either absent or in limited supply. The reactions and products involved in these three metabolically different pathways collectively make up the nitrogen cycle.
There are two ways in which organisms obtain ammonia. One is to use nitrogen already in a form easily metabolized to ammonia. Thus, nonviable plant, animal, and microbial residues in soil are enzymatically decomposed by a series of hydrolytic and other reactions to yield biosynthetic monomers such as amino acids and other small-molecular-weight nitrogenous compounds. These amino acids, purines, and pyrimidines are decomposed further to produce NH3 which is then used by plants and bacteria for biosynthesis, or these biosynthetic monomers can be used directly by some microorganisms. The decomposition process is called ammonification.
The second way in which inorganic nitrogen is made available to biological agents is by nitrogen fixation (this term is maintained even though N2 is now called dinitrogen), a process in which N2 is reduced to NH3. Since the vast majority of nitrogen is in the form of N2, nitrogen fixation obviously is essential to life. The N2-fixing process is confined to prokaryotes (certain photosynthetic and nonphotosynthetic bacteria). The major nitrogen fixers (called diazotrophs) are members of the genus Rhizobium, bacteria that are found in root nodules of leguminous plants, and of the cyanobacteria (originally called blue-green algae).