oestrogen
(US), estrogenEstrogen
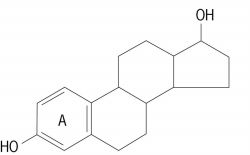
A substance that maintains the secondary sex characters and organs, such as mammary glands, uterus, vagina, and fallopian tubes, of mammalian females. Naturally occurring substances with this activity are steroid hormones. The principal estrogenic hormone substances are 17(ß)-estradiol (with the structure shown), estrone, and estriol. They are produced and secreted directly into the bloodstream by the ovary, testis, adrenal, and placenta of pregnancy. Two other naturally occurring estrogenic hormones, equilin and equilenin, have been obtained only from the urine of pregnant mares and are apparently peculiar to that species. Stilbestrol, a synthetic compound with considerable estrogenic activity, has been used extensively in medical practice. See Hormone, Steroid
estrogen
[′es·trə·jən]Estrogen
any of a number of female sex hormones (including estradiol, estriol, and estrone) produced mainly by the ovarian follicles and placenta and to some extent by the adrenal cortex and testes.
Steroids in chemical structure, estrogens stimulate the growth and development of the genitalia and secondary sex characteristics in women and in the females of many mammals. Estrogens contain 18 carbon atoms and possess the chemical properties of phenols. The biosynthesis of these steroid hormones in mammals is one of the final stages in the metabolism of cholesterol. The closest biogenetic precursors of estrogens are androgens (male sex hormones).
Estrogens occur in the blood in complexes with proteins and are found in concentrations of less than 10 micrograms/liter (μg/l) in both men and women. Estrogens are excreted from the body together with urine. In males estrogens are excreted evenly, but in females the process has two peaks—during ovulation and at the time of maximum activity of the yoke sac. During pregnancy and especially toward the end of the term, the level of estrogens in women’s blood rises to 70–80 μg/l as a result of the marked intensification of the biosynthesis of the hormone in the placenta. Estrogens cause thickening of the epithelium of the vaginal mucosa, an increase in the weight of the uterus, the rhythmic contraction of the uterus, stimulation of the alveolar tissue of the breasts, formation of a layer of subcutaneous fat, the growth of hair of the female type, and a persistent sex drive. Together with the hormone progesterone, they facilitate the implantation of the fertilized egg, help maintain pregnancy, and ease childbirth. Estrogens play an important role in the regulation of many other biochemical processes by participating in the metabolism of carbohydrates and the distribution of lipids, stimulating the synthesis of amino acids, nucleic acids, and proteins, aiding the deposition of calcium in bone tissue, and delaying the excretion of sodium, inorganic phosphorus, and water.
The secretion of estrogens is controlled by the anterior pituitary and its gonadotropic hormones—the follicle-stimulating hormone (FSH) and the luteinizing hormone (LH). The biochemical mechanism of the action of steroid hormones is probably related to the stimulation of the synthesis of ribonucleic acid (RNA) in the cells and tissues of the reproductive organs, which causes a change in the rate and volume of protein biosynthesis (seeTRANSLATION).
Synthetic steroid derivatives of estrogens (including estradiol dipropionate, estradiol benzoate, and ethinyl estradiol) are used to treat such problems as hyperplasia of the genitalia in women, disturbances of the menstrual cycle, climacteric disorders, and certain diseases affecting the sexual organs in female mammals. Considerable estrogenic activity is also exhibited by such other compounds as synthetic nonsteroid derivatives of 1,2-diphenyl-ethylene, including diethylstilbestrol, hexestrol, and synestrol; of triphenylethylene, including chlorotrianisene and bromotriphenylethylene; and of α-naphthylpropionic acid, including allenoic acid and 3-(5-naphthol)-4,4-dimethylpentanoic acid.
Estrogens are found not only in humans and animals but also in some higher plants, especially in certain flowers (such as the flower of the willow) and fruits (such as apples, coconuts, dates, and pomegranates), but their function and metabolism is still obscure. Such microorganisms as Nocardia restricta and Septomyxa offinis can also synthesize estrogens from sterols. Many legumes, including clover, soya, and alfalfa, form oxygen-containing heterocyclic compounds with high estrogenic activity; such compounds include coumestrol, genistein, and mirestrol.
REFERENCES
Pfizer, L., and M. Pfizer. Steroidy. Moscow, 1964. (Translated from English.)Heftman, E. Biokhimiia steroidov. Moscow, 1972. (Translated from English.)
E. P. SEREBRIAKOV