Free energy
A term in thermodynamics which in different treatments may designate either of two functions defined in terms of the internal energy E or enthalpy H, and the temperature-entropy product TS.
The function (E – TS) is the Helmholtz free energy and is the function ordinarily meant by free energy in European references. The Gibbs free energy is the function (H – TS). For the Lewis and Randall school of American chemical thermodynamics, this is the function meant by the free energy F. To avoid confusion with the symbol F as applied elsewhere to the Helmholtz free energy, the symbol G has also been used. Another development was the introduction of the name free enthalpy, with symbol G, for the Gibbs function. See Work function (thermodynamics)
For a closed system (no transfer of matter across its boundaries), the work which can be done
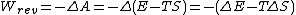
For constant temperature and pressure in a reversible process the decrease in the Gibbs function G for the system again corresponds to a free-energy change in the above sense, since it is equal to the work which can be done by the closed system other than that associated with its change in volume ΔV under the given constant pressure P. The relations shown in Eq. (2) can be formed since ΔH = ΔE + PΔV.
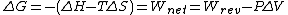
Each of these free-energy functions is an extensive property of the state of the thermodynamic system. For a specified change in state, both ΔA and ΔG are independent of the path by which the change is accomplished. Only changes in these functions can be measured, not values for a single state.
The thermodynamic criteria for reversibility, irreversibility, and equilibrium for processes in closed systems at constant temperature and pressure are expressed naturally in terms of the function G. For any infinitesimal process at constant temperature and pressure, -dG ≥ δwnet. If δwnet is never negative, that is, if the surroundings do no net work on the system, then the change dG must be negative or zero. For a reversible differential process, -dG > δwnet; for an irreversible process, -dG > δwnet. The free energy G thus decreases to a minimum value characteristic of the equilibrium state at the given temperature and pressure. At equilibrium, dG = 0 for any differential process taking place, for example, an infinitesimal change in the degree of completion of a chemical reaction. A parallel role is played by the work function A for conditions of constant temperature and volume. Because temperature and pressure constitute more convenient working variables than temperature and volume, it is the Gibbs free energy which is the more commonly used in thermodynamics. See Entropy, Thermodynamic principles
free energy
[¦frē ′en·ər·jē]Free Energy
a term used to refer to the Gibbs function and to the Helmholtz function, both of which are thermodynamic potentials. In Soviet usage, the term “free energy” is used primarily for the Helmholtz function.
The Helmholtz free energy, also called the work function, is defined as the difference between the internal energy U of a thermodynamic system and the product of the system’s entropy S and temperature T. The quantity ST, which is subtracted from the internal energy when the free energy is found, is sometimes called the bound energy.