Heat capacity
The quantity of heat required to raise a unit mass of homogeneous material one unit in temperature along a specified path, provided that during the process no phase or chemical changes occur, is known as the heat capacity of the material in question. Moreover, the path is so restricted that the only work effects are those necessarily done on the surroundings to cause the change to conform to the specified path. The path is usually at either constant pressure or constant volume.
In accordance with the first law of thermodynamics, heat capacity at constant pressure Cp is equal to the rate of change of enthalpy with temperature at constant pressure ∂H/∂T)p. Heat capacity at constant volume Cv is the rate of change of internal energy with temperature at constant volume (∂U/∂T)v. Moreover, for any material, the first law yields the relation 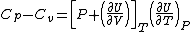 See Enthalpy, Internal energy, Thermodynamic principles
See Enthalpy, Internal energy, Thermodynamic principles
heat capacity
[′hēt kə‚pas·əd·ē]Heat capacity
The quantity of heat required to raise a unit mass of homogeneous material one unit in temperature along a specified path, provided that during the process no phase or chemical changes occur, is known as the heat capacity of the material in question. Moreover, the path is so restricted that the only work effects are those necessarily done on the surroundings to cause the change to conform to the specified path. The path is usually at either constant pressure or constant volume.
In accordance with the first law of thermodynamics, heat capacity at constant pressure Cp is equal to the rate of change of enthalpy with temperature at constant pressure. Heat capacity at constant volume Cv is the rate of change of internal energy with temperature at constant volume. Moreover, for any material, the first law yields the relation
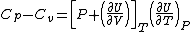
See Enthalpy, I<SCP>nternal energy</SCP>, T<SCP>hermodynamic principles</SCP>.
heat capacity, thermal capacity
Heat Capacity
(or thermal capacity), the quantity of heat absorbed by a substance when its temperature is raised 1 degree. Instantaneous heat capacity is the ratio of the heat absorbed by a substance, upon an infinitesimal change in its temperature, to the change in temperature. The heat capacity per unit mass (in, for example, g or kg) of a substance is called the specific heat. The heat capacity per mole of a substance is known as the molar, mo-lal, or molecular heat capacity.
The quantity of heat absorbed by a substance when a change of state occurs depends not only on the initial and final states—in particular, on their temperature—but also on the means by which the transition between them was accomplished. Accordingly, the heat capacity of a substance depends on the method of heating. A distinction is usually made between the heat capacity at constant volume (cv) and the heat capacity at constant pressure (cp) depending on whether the volume or pressure, respectively, is held constant during the heating process. In the case of heating at constant pressure, part of the heat provides the energy needed to do the work of expanding the substance, and part goes to increase the internal energy of the substance. For heating at constant volume, all the heat goes to increase the internal energy. Consequently, cp is always greater than cv. For gases that are so rarified that they may be regarded as ideal, the difference in molar heat capacities cp – cv is equal to R, the universal gas constant, which is equal to 8.314 joules per mole degree Kelvin (J/mol °K), or 1.986 calories per mole degree Celsius (cal/mol °C). In liquids and solids, the difference between cp and cv is comparatively small.
The theoretical calculation of heat capacity—in particular, the calculation of the dependence of the heat capacity on the temperature of the substance—cannot be carried out by means of purely thermodynamic methods and requires application of the methods of statistical mechanics. For gases, the calculation of heat capacity reduces to calculating the average energy of the thermal motion of the individual molecules. This motion consists of the translational and rotational motions of the molecule as a whole and of the vibrational motion of the atoms within the molecule.
According to classical statistics—that is, statistical mechanics based on classical mechanics—each degree of freedom of translational and rotational motion contributes the amount R/2 to the molar heat capacity (cv) of a gas, and each vibrational degree of freedom contributes the amount R. This principle is called the equipartition of energy. A particle of a monatomic gas has three translational degrees of freedom. Accordingly, the molar heat capacity of the gas should be 3R/2 (that is, about 12.5 J/mol °K, or 3 cal/mol °C), which is in good agreement with experiment. A molecule of a diatomic gas has three translational, two rotational, and one vibrational degree of freedom, and the equipartition law yields the value cv = 7R/2.
Experiment, however, shows that the molar heat capacity of a diatomic gas at ordinary temperatures is actually 5R/2. The reason for this discrepancy between theory and experiment is that in calculating the molar heat capacity quantum effects must be taken into account—that is, a statistics based on quantum mechanics must be used. According to quantum mechanics, any system of particles that execute vibrations or rotations (including a gas molecule) can have only certain discrete energy values. If the energy of thermal motion in the system is insufficient to excite vibrations of a certain frequency, then these vibrations do not contribute to the heat capacity of the system; the corresponding degree of freedom is “frozen”—that is, the equipartition law is inapplicable.
The temperature T at which the equipartition law is applicable to rotational or vibrational degrees of freedom is determined by the quantum-mechanical relation T ≫ hvlk, where v is the frequency of the vibrations, h is Planck’s constant, and k is the Boltzmann constant. The intervals between the rotational energy levels of a diatomic molecule (divided by k) are just a few degrees and reach a hundred degrees only for such a light molecule as the hydrogen molecule. At ordinary temperatures, the rotational part of the heat capacity of diatomic (and sometimes polyatomic) gases therefore obeys the equipartition law. By contrast, the intervals between vibrational energy levels reach a few thousand degrees. At ordinary temperatures, the equipartition law is consequently inapplicable to the vibrational part of the heat capacity. The calculation of heat capacity on the basis of quantum statistics indicates that the vibrational part of heat capacity decreases rapidly with decreasing temperature and approaches zero. For this reason, even at ordinary temperatures the vibrational part of heat capacity is practically absent, and the molar heat capacity of a diatomic gas is 5R/2 instead of 7R/2.
At sufficiently low temperatures, heat capacity must in general be calculated by means of quantum statistics. The heat capacity decreases with decreasing temperature and approaches zero as T → 0, in accordance with the Nernst heat theorem (the third law of thermodynamics).
In solids (crystalline substances) the thermal motion of the atoms occurs as small vibrations near certain equilibrium positions (lattice points). Each atom thus has three vibrational degrees of freedom. According to the equipartition law, the molar heat capacity of a solid—that is, the heat capacity of the crystal lattice—should be equal to 3nR, where n is the number of atoms in the molecule. In actuality, however, this value is the limit approached by the molar heat capacity of a solid at high temperatures. The limit is reached at ordinary temperatures in many elements, including the metals, for which n = 1 and the Dulong and Petit law applies. It is also reached in some simple compounds, such as NaCl and MnS, for which n = 2, and PbCl2, for which n = 3. In complex compounds the limit is never actually reached, since melting or decomposition of the substance begins before the limit can be attained.
The quantum theory of the heat capacity of solids was developed by A. Einstein in 1907 and P. Debye in 1912. The theory is based on the quantization of the vibrational motion of the atoms in a crystal. At low temperatures, the specific heat of a solid is proportional to the cube of the absolute temperature; this principle is known as the Debye T3 law. High and low temperatures can be distinguished through comparison with the parameter characteristic of each individual substance called the characteristic, or Debye, temperature θD. This quantity is determined by the vibration spectrum of the atoms in the substance and thus depends essentially on the crystal structure. Although θD is usually of the order of a few hundred °K, it can reach thousands of °K—for example, in diamond (seeDEBYE TEMPERATURE).
In metals, conduction electrons also make a definite contribution to heat capacity. This part of heat capacity can be calculated by means of Fermi statistics, which electrons obey. The electronic specific heat of a metal is proportional to the absolute temperature. A comparatively small quantity, the electronic contribution to the specific heat of a metal becomes considerable at temperatures close to absolute zero: at temperatures of the order of a few degrees, the specific heat associated with the vibrations of atoms in the crystal lattice is a still smaller quantity.
| Table 1. Specific heats of some substances | |
|---|---|
| Substance | Specific heat (kcal/kg °C) |
| Nitrogen ............... | 0.249 |
| Hydrogen ............... | 3.42 |
| Iron ............... | 0.104 |
| Copper ............... | 0.091 |
| Aluminum ............... | 0.210 |
| Lead ............... | 0.030 |
| Quartz ............... | 0.174 |
| Ethyl alcohol ............... | 0.547 |
| Water ............... | 1.008 |
Table 1 gives the specific heats, in kilocalories per kilogram degree, of some gases, liquids, and solids at a temperature of 0°C and at atmospheric pressure (1 kilocalorie is equal to 4.19 kilojoules).
REFERENCES
Kikoin, I. K., and A. K. Kikoin. Molekuliarnaia fizika. Moscow, 1963.Landau, L. D., and E. M. Lifshits. Statislicheskaia fizika, 2nd ed. (Teoreticheskaia fizika, vol. 5.) Moscow, 1964.
E. M. LIFSHITS