Magnetic resonance
A phenomenon exhibited by the magnetic spin systems of certain atoms whereby the spin systems absorb energy at specific (resonant) frequencies when subjected to alternating magnetic fields. The magnetic fields must alternate in synchronism with natural frequencies of the magnetic system. In most cases the natural frequency is that of precession of the bulk magnetic moment of constituent atoms or nuclei about some magnetic field. Because the natural frequencies are highly specific as to their origin (nuclear magnetism, electron spin magnetism, and so on), the resonant method makes possible the selective study of particular features of interest. For example, it is possible to study weak nuclear magnetism unmasked by the much larger electronic paramagnetism or diamagnetism which usually accompanies it.
Nuclear magnetic resonance (that is, resonance exhibited by nuclei) reveals not only the presence of a nucleus such as hydrogen, which possesses a magnetic moment, but also its interaction with nearby nuclei. It has therefore become a most powerful method of determining molecular structure. The detection of resonance displayed by unpaired electrons, called electron paramagnetic resonance, is also an important application. See Magnetic resonance, Magnetism
magnetic resonance
[mag′ned·ik ¦rez·ən·əns]Magnetic Resonance
the selective absorption by a substance of electromagnetic waves of a particular wavelength as a result of a change in the orientation of the magnetic moments of electrons or atomic nuclei. In an external magnetic field the energy levels of a particle that has a magnetic moment JA are split into magnetic substates, each of which corresponds to a certain orientation of the magnetic moment jn with respect to the field H. An electromagnetic field having a resonance frequency ω produces quantum transitions between the magnetic substates. The condition of resonance has the form
Δξ = ħ ω
where Δξ is the energy difference between the magnetic substates and ħ is Planck’s constant.
If electromagnetic energy is absorbed by the nuclei, the resonance is called nuclear magnetic resonance. The magnetic moments of nuclei are caused by their spin /. The number of nuclear magnetic substates is equal to 2I + 1 , and the intervals between adjacent substates are identical and equal to
Δξ = ħγH
where γ is the gyromagnetic ratio. The selection rules permit transitions only between adjacent substates; therefore, the same resonance frequency corresponds to all transitions (see Figure 1), the absorption lines overlap, and a single line is observed.
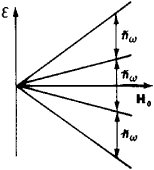
However, in some crystals for nuclei with spin I > 1 an additional shift of levels takes place because of the interaction of the nuclear quadrupole moment with the extranuclear nonuni form crystalline electric field E at the site of the nucleus. As a result, more lines appear in the absorption spectrum.
The magnetic resonance caused by the magnetic moments of electrons in paramagnets is called electron paramagnetic resonance. Its spectrum is a function of both the spin and the orbital motion of the electrons making up the paramagnetic atoms and molecules, and it is usually sensitive to the crystalline field at the site of the paramagnetic particle. In ferromagnets and antiferromagnets the electron magnetic resonances are called ferromagnetic and antiferromagnetic resonance, respectively.
In many cases the classic description of magnetic resonance is useful. It is based on the fact that in an external magnetic field H the magnetic moment μ of a particle experiences Larmor precession around the direction of the vector H at a frequency ω = γH. A variable magnetic field H1 perpendicular to H and rotating together with μ—that is, at a frequency ω—exerts a steady influence on the magnetic moment and changes its spatial orientation.
Cyclotron resonance, which is observed in metals and semiconductors situated in a constant magnetic field and is the resonance absorption of electromagnetic energy associated with the periodic motion of conduction electrons and holes in a plane perpendicular to the field H, is also classified as a magnetic resonance.
The frequency range of magnetic resonance depends on the value of the gyromagnetic ratio. For a free electron, γ/2π = 2.799 ×106 hertz-oersted1; for a proton, γ/2π = 4.257 ×103 hertz-oested-1 and for nuclei with spin, γ/2π = 102-103 hertz-oersted1. Accordingly, in magnetic fields of 103to 104 oersteds, the frequencies of electron paramagnetic resonances fall in the superhigh frequency range (109-1011 Hz), and the frequencies of nuclear magnetic resonance, in the shortwave range (106-107 Hz).
REFERENCES
Slichter, C. Osnovy teorii magnitnogo rezonansa. Moscow, 1967. (Translated from English.)Abragam, A. ladernyi magnetizm. Moscow, 1963. (Translated from English.)
Al’tshuler, S. A., and B. M. Kozyrev. Elektronnyi paramagnitnyi rezonans. Moscow, 1961.
V. A. ATSARKIN