hydrolysis
hydrolysis
[hī′dräl·ə·səs]Hydrolysis
an ion-exchange reaction between various substances and water. In general, hydrolysis is represented by the equation A—B + H—OH ⇄ A—H + B—OH, where A—B is the substance undergoing hydrolysis, and A—H and B—OH are the products of hydrolysis.
In hydrolysis of salts, equilibrium is governed by the law of mass action. If insoluble or readily volatile substances are formed by hydrolysis, there is virtually complete decomposition of the initial salt. In other cases, the weaker the acid or base of the salt, the more complete the hydrolysis.
If the salt of a weak acid and a strong base, such as KCN, is hydrolyzed, the solution is alkaline, because the anion of the weak acid partially binds the H+ ions formed by the dissociation of water, and the excess OH~ions remain in the solution:
K+ + CN− + HOH ⇄ HCN + K+ + OH−
A solution of a salt of a strong acid and a weak base, such as NH4Cl, is acidic (NH+4 + Cl− + HOH ⇄ NH4OH + H+ + Cl−). If the charge of the cation (or anion) of the salt exceeds 1, hydrolysis often yields acid (or basic) salts as the first-stage hydrolysis product—for example,
CuCl2 → Cu(OH)Cl → Cu(OH)2
The degree of hydrolysis (α) can be used as a quantitative characteristic for the hydrolysis of salts. It is equal to the ratio of the concentration of the hydrolyzed part of the molecules to the total concentration of the particular salt in solution, and in most cases it is small. Thus, with 0.1-molar solutions of sodium acetate, CH3COONa, or ammonium chloride, NH4Cl, at 25° C, α = 0.01 percent, whereas for ammonium acetate, CH3COONH4, α = 0.5 percent. The degree of hydrolysis increases with an increase in temperature and with dilution.
The hydrolysis of salts forms the basis of many important processes in the chemical industry and laboratory practice. The partial hydrolysis of tricalcium silicate causes separation of lime during the reaction of portland cement with water. Buffer systems, which are able to maintain constant acidity in a medium, exist because of hydrolysis. Buffer solutions are also very important physiologically, since a constant H+ion concentration is necessary for normal body activity. A number of geological changes in the earth’s crust and the formation of minerals, natural waters, and soils are associated with hydrolysis.
The hydrolysis of organic compounds is the decomposition of organic compounds by water, with the formation of two or more compounds. Hydrolysis is ordinarily effected in the presence of acids (acid hydrolysis) or alkalies (alkaline hydrolysis). The bond between a carbon atom and other atoms (halogen, oxygen, nitrogen, and so on) is most often dissolved by hydrolysis. Thus, alkaline hydrolysis of halides can be used to prepare alcohols and phenols, including those of industrial quality—for example,
![]()
Depending on the structure of the hydrocarbon radical (R) and the reaction conditions, hydrolysis of halogen derivatives can proceed as a monomolecular process (SNl) or a bimolecular process (SN2). In the case of a monomolecular reaction there is initial ionization of the carbon-halogen bond, followed by reaction of the resultant carbonium ion with water. If alkali is added it does not affect the rate of hydrolysis and serves only to neutralize the hydrohalic acid that is liberated and to shift the equilibrium:
![]()
In the bimolecular reaction, the rate of reaction is directly proportional to the alkali concentration:
R—Hal + HO → R—OH + Hal-SN2.
Ester hydrolysis (the reverse of esterification) is exceptionally important:
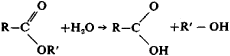
Acid hydrolysis of esters is reversible:
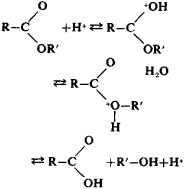
Alkaline hydrolysis of esters is irreversible, since it gives an alcohol and a salt of the acid:
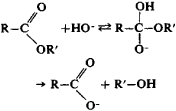
This process is used extensively in industry to make alcohols and acids—for example, in the saponification of fats to make glycerol and salts of higher acyclic acids (soaps). Amides are hydrolized analogously to esters:
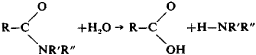
The hydrolysis of carbon-carbon bonds is comparatively rare. Particular examples are ketone decomposition (by acids and dilute alkalies) and acid decomposition (by concentrated alkalies) of 1,3-dicarbonyl compounds—for example, aceto-acetic ester:

In organic chemistry the term “hydrolysis” is also applied to certain processes that would more correctly be called hydration. An example is the conversion of nitriles to amides:
![]()
The hydrolysis of ester, glucoside (in carbohydrates), and amide (in proteins) bonds plays an important part in the vital activities of all organisms—that is, in processes such as the assimilation of food and the transmission of nerve impulses. In the living organism, hydrolysis is catalyzed by enzymes (hydrolases).
REFERENCES
Kireev, V. A. Kurs fizicheskoi khimii, 2nd ed. Moscow, 1956.Reutov, O. A. Teoreticheskie problemy organicheskoi khimii.2nd ed. Moscow, 1964.